Journal of Creation 38(1):60–66, April 2024
Browse our latest digital issue Subscribe
Late Pleistocene body size reduction: evidence of a post-Flood decline in longevity?
Even after the Flood, the Genesis patriarchs routinely experienced lifespans of hundreds of years (Genesis 11). Hence, biblical creationists should be interested in possible scientific corroboration for this extreme longevity. Whatever factor or factors enabled extreme human longevity also likely enabled greater animal longevity. Longevity studies have shown that greater longevity is often associated with larger adult body sizes and prolonged intervals of maturation. Hence, one might expect longer-lived people and animals to be larger than those with shorter lifespans. Thus, the ubiquity of giantism in the fossil record is noteworthy, as is evidence of decreasing body sizes during and after the post-Flood Ice Age. This evidence of body size reduction is worldwide and especially strong for mammals, but there is some evidence for body size reduction in other taxa. Because Pleistocene giantism was found even in places far from the high-latitude ice sheets, Bergmann’s rule, in and of itself, is likely an insufficient explanation. Because of the body size/longevity link, body size reduction is likely indirect evidence of declining longevity in the immediate post-Flood world.
Ancient humans routinely experienced lifespans of hundreds of years (Genesis 5 and 11), and creationists should be interested in possible scientific corroboration of this great longevity. Greater longevity in animals is often associated with larger adult body sizes and prolonged maturation intervals.1,2 The larger one’s adult body size and the greater the amount of time to attain that adult body size, the longer one’s lifespan will tend to be. Equivalently, reduced longevity is associated with smaller adult body masses and shorter maturation intervals (figure 1).
Most of the studies linking longevity to body size and maturation time have included organisms from across multiple higher taxonomic groupings; for example, families, classes, and/or orders within the Linnaean classification system. But of much greater interest to creationists is whether these trends hold within a particular Genesis ‘kind’ or baramin. Suppose a member of a particular baramin were raised under conditions that somehow caused it to attain to a much larger body size than other members of its baramin, and over a much longer time interval. Would the larger creature live longer?
The Genesis kind likely corresponds to the Linnaean genus or family.3 Hence, intra-species or intra-genus longevity-size correlations are almost guaranteed to apply within a Genesis kind. Though it is not as abundant, some such intra-species and intra-genus correlations do exist and are discussed in other papers.1,2 Also, a theory of ontogenetic growth predicts that the age at skeletal maturity, tmaturity, should be proportional to adult body mass, M, raised to the one-fourth power:4
tmaturity = kM¼ [1]
The derivation of Eq. (1) implicitly assumes that environmental conditions are held constant. So a change in those conditions could change the age at maturity, with a corresponding change in adult body mass, M.
Eq. (1) is in agreement with observations that biological timescales in general tend to be proportional to body mass raised to the ¼ power.5,6,7
Creationists have long noted the ubiquity of giantism in fossil creatures, and some have suggested a link between this giantism and longevity.8-15 Eq. (1) and the observations linking greater longevity to greater ages at maturity provide that linkage.
In this light, it is striking that 65 is the earliest age at which a Genesis 5 patriarch is listed as having a son (Genesis 5:15, 21). Although Genesis does not tell us whether or not the listed sons were firstborn, it seems likely that at least some of them were. Given the strength of the human sex drive, it seems very unlikely that the Genesis patriarchs were all deciding to postpone marriage for five decades! It is far more likely that they were becoming sexually mature at much greater ages than do humans today. Given the above discussion, one would expect very long-lived humans to take a greater amount of time to mature than humans with shorter lifespans. But this immediately begs another question: Were ancient humans bigger than we are? We briefly return to this question in the concluding remarks.
General evidence for post-Flood body reduction
If extant versions of creatures are smaller than the fossil versions then it is obvious that a reduction in size must have occurred, even if that reduction is not necessarily documented in the fossils themselves. However, in many cases the size reduction is revealed in the fossils. This paper describes both: general evidence for giantism and evidence that this giantism diminished or disappeared after the Flood.
There is very strong evidence for a ‘high’ Flood/post-Flood boundary in the rocks; generally speaking, high or higher than the Mid-Pleistocene.16,17,18,19 For this reason, this study confines itself primarily to evidence of body size reduction that occurred, by evolutionary reckoning, in the Late Pleistocene or Holocene. However, I also agree with Oard20 that Ice Age deposits will not necessarily always be classified as ‘Late Pleistocene’ on the uniformitarian timescale. Thus, a more thorough review of the stratigraphy surrounding the examples cited below could result in the removal of some of these examples, as well as the addition of some examples not included here.
Body reduction size in mammals
North America
Alaskan horse metacarpal bones decreased in length by 13.5% during the Late Pleistocene, implying a ‘dramatic’ and ‘unexpected’ body size decline prior to their extinction.21 Likewise, North American bighorn sheep are apparently the direct descendants of larger fossil sheep that underwent a body size reduction:
“A direct ancestor-descendant relationship between modern and the fossil sheep in North America seems probable. Reduction of body size seems likely to have occurred at the end of Pleistocene or the beginning of Holocene time.”22
Bighorn sheep are found in the North American great plains, and Oard has recently argued that Great Plains fossils may be Flood, rather than post-Flood.20 In any case, morphological shrinkage of North American bighorn sheep has occurred, and the same is true of North American bison, although there is disagreement among evolutionary scholars about the details.23
Extant North American bison (Bison bison) are comprised of the plains bison (B. bison bison) of the Great Plains and the wood bison (B. bison athabascae) of Canada and Alaska. Morphological shrinkage for the plains bison is illustrated24 in figure 2. Although some of the variation in horn size depicted in figure 2 could represent mere in-kind variation, analysis of post-cranial bison fossil data also implies that a true body size reduction did occur.25 The bison shown in figure 2 are in stratigraphic sequence, although evolutionists do not necessarily believe that all the bison shown are in a direct lineage. A giant version of the steppe bison Bos priscus (figure 3) is thought to have come to North America from Eurasia via the Bering Land Bridge, becoming the ancestor of B. latifrons. Some claim that B. antiquus is descended from B. latifrons, as is B. occidentalis.26 In turn, extant bison B. bison are descended from B. antiquus,27 possibly via hybridization with B. occidentalis:
“Likely, B. antiquus and B. occidentalis did not go extinct, but through phenotypic and morphologic adaptation to changing climatic conditions, evolved into what is traditionally referred to as B. bison that we have throughout the Holocene … .”28
Interestingly, Late Pleistocene fossils of smaller B. priscus bison are also found in North America, suggesting that this larger steppe bison also became smaller over time.29
The Pleistocene and Holocene glyptodont Doedicurus sp. was a one-ton armadillo-like creature (figure 4), recently shown by DNA analysis to actually be a genuine armadillo.30,31 Late Pleistocene North America was home to the giant beavers Castoroides dilophidus and Castoroides ohioensis. Despite their claim that modern and giant beavers were unrelated, evolutionists have acknowledged, “Even so, the shapes of their bones look a lot like those of a modern beaver, only much larger.”32
Europe and Asia
Fossil evidence implies the extant wild boar (Sus scrofa) underwent a Holocene or Late Pleistocene body size reduction in both Italy and Japan.33,34 The European badger (Meles meles) in southern Italy also apparently became smaller in size during the Late Pleistocene.35 Walvius noted that western European red deer are about one third smaller than their ‘neolithic’ forebears.36
The Middle to Late Pleistocene Palaeoloxodon namadicus from India37 is clearly a member of the Genesis ‘elephant kind’, but it was much larger than extant elephants (figure 5), and the giant beaver Trogontherium cuiveri lived in Late Pleistocene China.38
Davis (1981) cites evidence that, on the uniformitarian timescale, the majority of large animals in Israel, including foxes, wolves, boars, aurochs, and goats, became smaller 12,000 years ago, and some animals then underwent another size reduction due to domestication.39
In 2006 bones of an enormous Late Pleistocene camel, “double the size of a modern-day camel” were unearthed near the village of El Kowm in Syria.40 In the Late Pleistocene, giant camels lived in Mongolia,41,42 and the giant ape Gigantopithecus blackii is thought to have inhabited Southeast Asia.43 In passing it is worth noting that fossils of giant camels have also been found in North America, although these specimens are found in rocks that may be from the Flood.44
South America
Although South America has no extant megafauna, many large mammals existed in Pleistocene South America that can aptly be called giants:
“Bears, sabertooth cats, enormous capybaras, and llamas roamed across South America, as well as other bizarre creatures including massive terrestrial sloths, armored glyptodonts (hippo-sized animals closely related to armadillos), and peculiar animals reminiscent of camels and rhinoceroses (macrauchenids and toxodonts). These enigmatic animals were decimated during the Quaternary—all South American mammal species larger than 100 kg were lost. The mystery surrounding their extinction has yet to be fully resolved, and is a topic of considerable debate … .”45
These extinct South American megafauna, thought to have survived until the mid-Pleistocene or Holocene, include the giant ground sloth Megatherium46 and the giant glyptodon armadillos47 (figure 6).
Africa
Pleistocene and early Holocene Africa, too, had larger versions of extant creatures, including the bovine Megalotragus, with body similarities to the extant hartebeest and wildebeest in the bovid subfamily Alcelaphinae.48 It is not difficult to imagine that Megalotragus was simply a larger ancestor of these smaller extant alcelaphines. Pleistocene Africa was home to other megafauna, such as Dinopithecus ingens (‘gigantic terrible ape’) twice the size of an extant baboon,49 the East African lion Panthera leo,50 and the giant Olduvai buffalo Pelorovis oldowayensis.51 These last three examples are Early- or Mid-Pleistocene and thus may represent Flood fossils. However, it is not difficult to imagine that extant African species are descended from these larger animal forms. Moreover, Beasley suggested that evolutionists could be mistaking giant African fossil apes for alleged ‘ape-men’.12
Australia
Some Australian megafauna are likely the ancestors of smaller extant fauna. Oard and Arment agree that Australian marsupial fossils (excluding those from the early Cenozoic) are post-Flood.20,52,53 These included the giant wombat Phascolonus gigas. Extant wombats still live in Australia, but they are much smaller. Australia was also once home to giant kangaroos, including the giant short-faced kangaroo Procoptodon goliah (figure 7), and Macropus titan, very similar to the extant eastern grey kangaroo, except for its 30% larger size.54 The giant wallaby Protemnodon anak, named after the biblical giants the Anakim (Deuteronomy 2:11, 21, 9:2), also lived in Late Pleistocene Tasmania.55
In light of this worldwide data, it is hardly surprising that Davis wrote:
“[Finnish paleontologist Björn] Kurtén (1965) discovered that most carnivores in Israel and Lebanon underwent a considerable size reduction at the end of the Pleistocene. The dwarfing of fossil mammal lineages at the end of the late Quaternary was probably world-wide” [emphasis added].39
Counterexamples
Of course, there are counterexamples to this trend. Mountain gazelles in the Levant apparently become larger from the Late Pleistocene to Early Holocene.56 Likewise, aurochs (Bos primigenius) in Italy became smaller from the Late Pleistocene through to the Holocene, but this was preceded by an apparent increase in size from the Early to Mid-Pleistocene.57 I believe this is likely an artifact due to the chaotic nature of the Flood, but it could be argued that this is evidence against this paper’s thesis. Likewise, there are examples of island dwarfism during the Pleistocene,58,59 including some examples from the Early and Mid-Pleistocene.60 Likewise, Davis reported that recent gazelles in northern Israel were larger than Early Holocene specimens.39 Nevertheless, a Late Pleistocene body size reduction seems to be the general rule.
Bergmann’s Rule not an adequate explanation
Bergmann’s Rule61 asserts that mammals and birds, and perhaps other organisms, living at higher, colder latitudes tend to be larger than organisms living at lower latitudes, presumably as an adaption for conserving body heat. Since the Genesis Flood caused an Ice Age,62 this seems at first glance to be a reasonable explanation for both large mammal body sizes during the Ice Age and the diminution of those body sizes as the Ice Age came to an end. However, Bergmann’s rule is somewhat controversial63 and is not a completely adequate explanation even if it is correct. Bergmann’s rule can perhaps explain some post-Flood giantism, but it does not explain the pre-Flood giantism of creatures which both creationists and evolutionists agree were living under warmer climatic conditions, such as dinosaurs in the pre-Flood world. Nor does it explain, in the creation model, the large sizes of Ice Age animals living far from the high-latitude ice sheets, since the Flood Ice Age model posits a more equable climate.64 It seems more likely that Bergmann’s rule is a second-order effect modulating body size, with some other, more fundamental cause (increased longevity?), resulting in giantism in general.65
Body size reduction in other taxa?
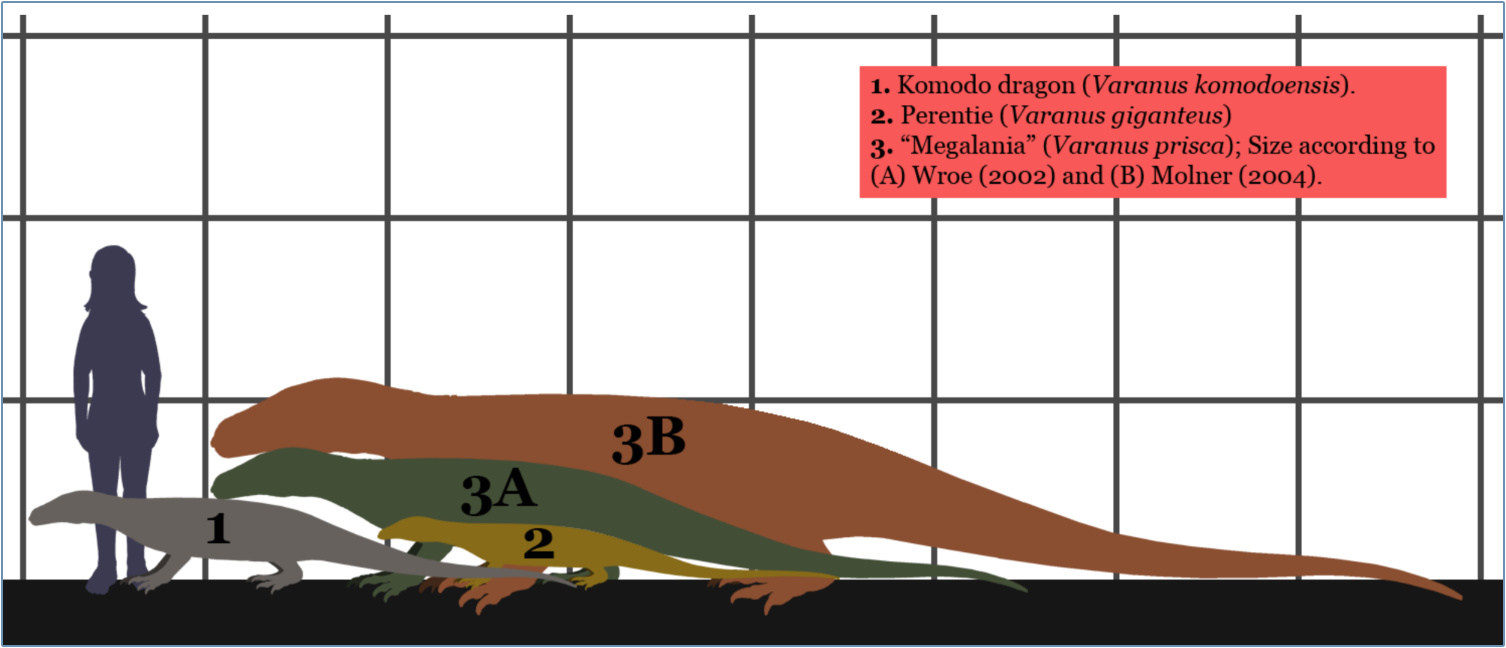
There is some evidence that other taxa also underwent a size reduction toward the end of the Ice Age. The giant monitor lizard Megalania (or Varanus priscus) lived in Australia during the Late Pleistocene,66 dwarfing even the extant Varanus giganteus and Komodo dragon Varanus komodoensis (figure 8).67,68 South African fur seals, angulate tortoises and granite limpets underwent an apparent size reduction from the Middle Stone Age (250 to 200 ka) to the Later Stone Age (50 to 40 ka).69 Likewise, the southern African tick shell, Nassarius kraussianus, used by early humans for ornamentation, decreased in size from the Pleistocene to the Holocene, prompting evolutionists to ask, Why were the shells of mankind’s earliest ornament larger in the Pleistocene than in the Holocene?70
Concluding remarks
Given the trends noted in the introduction, a general decrease in animal body size makes sense if longevity was decreasing during the post-Flood Ice Age. This was suggested by Greg Beasley in this very journal more than thirty years ago:
“The fossilised remains of both flora and fauna are, as a rule, significantly larger in the past than in their extant counterparts. One possible explanation for this ‘shrinkage’ over time is that the growth potential of living organisms has been impeded through earlier maturation and declining longevity; a consequence of changes in the prevailing biospheric conditions during the earth’s recent past. It is proposed that these changes were brought about by, and as a consequence of, geophysical, atmospheric and biological changes, initiated during the Flood. The writer proposes that morphological shrinkage is primarily a phenomenon of the post-Flood period, as was declining longevity and earlier skeletal maturation [bold added, italics original emphasis].”12
I believe Beasley was absolutely correct. What is more, the budding field of sclerochronology is now providing both indirect and direct evidence in support of his proposal, only some of which has as of yet been discussed in the creation literature.1,2
It is worth noting that large and small animals are often found together at Late Pleistocene sites.36,71,72 Carter has noted that very old members of a population tend to be far less numerous than younger members of that population.73 Could it be that the smaller animals were juveniles living alongside the larger adults?
Of course, as noted at the beginning of this paper, this discussion absolutely begs the question, Did humans, like animals, also undergo a body size reduction after the Flood? There is much scientific and cultural evidence that they did, which I briefly discussed in a recent short article.74 However, that subject deserves a much more in-depth treatment that must await a separate paper.
References and notes
- Hebert III, L., Allometric and metabolic scaling: Arguments for design … and clues to explaining pre-Flood longevity?, in: Proc. 9th ICC, Cedarville University, OH, pp. 206–227, 2023. Return to text.
- Hebert, J., Sherwin, F.J., and Overman, R., Crassostrea oysters: evidence for extreme longevity in the pre-Flood world?, CRSQ, in press. Return to text.
- Woodmorappe, J., Noah’s Ark: A feasability study, Institute for Creation Research, Santee, CA, 1996. Return to text.
- West, G.B., Brown, J.H., and Enquist, B.J., A general model for ontogenetic growth, Nature 413 (6856):628–631, 2001. Return to text.
- Calder, W.A. III., Size, Function, and Life History, Harvard University Press, Cambridge, MA, 1984. Return to text.
- Lindstedt, S., Body size, physiological time, and longevity of homeothermic animals, The Quarterly Review of Biology 56(1):1–16, 1981. Return to text.
- Schmidt-Nielsen, K., Scaling: Why is animal size so important?, Cambridge University Press, Cambridge, 1986. Return to text.
- Woetzel, D., Chronicles of Dinosauria, Dobbs, R. illust., Master Books, Green Forest, AR, 2013. Return to text.
- Coppedge, D.F., Life is devolving from a past world of giants; crev.info, accessed 20 Mar 2023. Return to text.
- Nelson, V., Monumental Monsters, Untold Secrets of Planet Earth Publishing Company, Red Deer, Alberta, Canada, 2017. Return to text.
- Patten, D.W., The longevity accounts in ancient history, CRSQ 19(1):40–52, 1982. Return to text.
- Beasley, G., Pre-Flood giantism: a key to the interpretation of fossil hominids and hominoids, J. Creation 4(1):5–55, 1990. Return to text.
- Taylor, P.S., The Great Dinosaur Mystery and the Bible, Master Books, El Cajon, CA, 1987. Return to text.
- Cuozzo, J.W., Neandertal children’s fossils: reconstruction and interpretation distorted by assumptions, J. Creation 8(2):166–178, 1994. Return to text.
- Line, P., Explaining robust humans, J. Creation 27(3):64–71, 2013. Return to text.
- Holt, R.D., Evidence for a late Cainozoic Flood/post-Flood boundary, J. Creation 10(1):128–167, 1996. Return to text.
- Oard, M.J., Dealing carefully with the data, J. Creation 16(1):68–72, 2002. Return to text.
- Oard, M.J., Geology indicates the terrestrial Flood/post-Flood boundary is mostly in the late Cenozoic, J. Creation 27(1):119–127, 2013. Return to text.
- Clarey, T.L., Werner, D.J., and Tomkins, J.P., Globally extensive Cenozoic coals indicate high post-Flood boundary, J. Creation 36(1):13–15, 2022. Return to text.
- Oard, M.J., The Central and Southern High Plains animals likely buried in the Flood, CRSQ 59(4):228–240, 2023. Return to text.
- Guthrie, R.D., Rapid body size decline in Alaskan Pleistocene horses before extinction, Nature 426(6963):169–171, 2003. Return to text.
- Wang, X., Systematics and population ecology of Late Pleistocene Bighorn Sheep (Ovis canadensis) of Natural Trap Cave, Wyoming, Transactions of the Nebraska Academy of Sciences XVI:173–183 (esp. p. 173), 1988. Return to text.
- Martin, J.M., Mead, J.I., and Barboza, P.S., Bison body size and climate change, Ecology and Evolution 8:4564–4574, 2018. Return to text.
- Schultz, C.B. and Hillerud, J.M., Climatic change and the extinction of large mammals during the Quaternary, Transactions of the Nebraska Academy of Sciences and Affiliated Societies VI:95–105, 1978. Return to text.
- Martin, J.M., Mead, J.I., and Barboza, P.S., Bison body size and climate change, Ecology and Evolution 8:4564–4574, 2018. Return to text.
- Berg, F. and Fink, R., The incredible shrinking buffalo–buffalo tales and trails, buffalotalesandtrails.com, accessed 10 Aug 2022. Return to text.
- Wilson, M.C., Hills, L.V., and Shapiro, B., Late Pleistocene northward-dispersing Bison antiquus from the Bighill Creek Formation, Gallelli Gravel Pit, Alberta, Canada, and the fate of Bison occidentalis, Canadian J. Earth Sciences 45(7):827–859, 2008. Return to text.
- Martin et al., ref. 25, p. 4,565. Return to text.
- Froese, D., Stiller, M., Heintzman, P.D. et al., Fossil and genomic evidence constrains the timing of bison arrival in North America, PNAS 114 (13):3457–3462, 2017. Return to text.
- Anonymous, Extinct glyptodonts really were gigantic armadillos, ancient DNA shows, Phys.org, 22 Feb 2016. Return to text.
- Delsuc, F., Gibb, G.C., Kuch, M. et al., The phylogenetic affinities of the extinct glyptodonts, Current Biology 26(4):155–156, 2016. Return to text.
- Giant beaver | Yukon Beringia Interpretive Centre; beringia.com, accessed 6 Oct 2023. Return to text.
- Iannucci, A., Sardella, R., Strani, F., and Mecozzi, B., Size shifts in late Middle Pleistocene to Early Holocene Sus scrofa (Suidae, Mammalia) from Apulia (southern Italy): ecomorphological adaptations?, Hystrix 31(1):10–20, 2020. Return to text.
- Fujita, M., Yamasaki, S., Sugawara, H., and Eda, M., Body size reduction in wild boar (Sus scrofa) from the late Pleistocene Maehira Fissure Site in Okinawajima Island, Japan, with relevance to human arrival, Quaternary International 339–440:289–299, 2014. Return to text.
- Iurino, D.A., Body size reduction and tooth agenesis in Late Pleistocene Meles meles (Carnivora, Mammalia) from Ingarano (Southern Italy), Rivista Italiana di Paleontologia e Stratigrafia 120(1):109–118, 2014. Return to text.
- Walvius, M.R., A discussion of the size of recent red deer (Cervus elaphus L.) compared with prehistoric specimens, Beaufortia 97(9):75–82, 1961. Return to text.
- Jukar, A.M., Lyons, S.K., Wagner, P.J., and Uhen, M.D., Late Quaternary extinctions in the Indian subcontinent, Palaeogeography, Palaeoclimatology, Palaeoecology 562:110137, 2021. Return to text.
- Yan, Y., Li, Q., Fostowicz-Frelik, Ł., and Ni, X., Last record of Trogontherium cuvieri (Mammalia, Rodentia) from the late Pleistocene of China, Quaternary International 513:30–36, 2019. Return to text.
- Davis, S.J.M., The effects of temperature change and domestication on the body size of Late Pleistocene to Holocene mammals of Israel, Paleobiology 7(1):101–114, 1981. Return to text.
- Anonymous, Giant camel fossil found in Syria; bbc.co.uk, accessed 10 Oct 2006. Return to text.
- Anonymous, Last of the giant camels and archaic humans lived together in Mongolia until 27,000 years ago, Phys.org, 24 Mar 2022. Return to text.
- Klementiev, A.M., Khatsenovich, A.M., Tserendagva, Y. et al., First documented Camelus knoblochi Nehring (1901) and Fossil Camelus ferus Przewalksi (1878) from Late Pleistocene archaeological contexts in Mongolia, Frontiers in Earth Science 10, Article 861163, 2022. Return to text.
- Carey, B., Gigantic apes coexisted with early humans, study finds, LiveScience, 7 Nov 2005. Return to text.
- Barbour, E.H. and Schultz, C.B., A new giant camel, Titanotylopus nebraksensis, gen. et sp. nov, Bulletin of the Nebraska State Museum 1(36):291–294, 1934. Return to text.
- Samonds, K.E., Book Review: Megafauna: Giant Beasts of Pleistocene South America, by Fariña, R.A., Vizcaíno, S.F., and Iuliis, G.D., J. Mammalogy 95(6):1308–1309, 2014. Return to text.
- Zurita, A.E., Carlini, A.A., Scillato-Yané, G.J., and Tonni, E.P., The extinct mammals of the Quaternary of Chaco Province, Argentina, and its relationship with those of the east of the pampean area and Chile, Revista Geológica de Chile 31(1):65–87, 2004. Return to text.
- Mitchell, K.J., Scanferla, A., Soibelzon, E. et al., A., Ancient DNA from the extinct South American giant glyptodont Doedicurus sp. (Xenarthra: Glyptodontidae) reveals that glyptodons evolved from Eocene armadillos, Molecular Ecology 25(14):499–508, 2016. Return to text.
- Brink, J.S., The evolution of the black wildebeest, Connochaetes gnou, and modern large animal faunas in central southern Africa, Ph.D. dissertation, University of Stellenbosch, pp. 107, 125, 2005. Return to text.
- Delson, E., Terranova, C.J., Jungers, W.L. et al., Body mass in Cercopithecidae (Primates, Mammalia): estimation and scaling in extinct and extant taxa, American Museum of Natural History Anthropological Papers 83:1–159, 2000. Return to text.
- Manthi, F.K., Brown, F.H., Plavcan, M.J., and Werdelin, L., Gigantic lion, Panthera leo, from the Pleistocene of Natodomeri, eastern Africa, J. Paleontology 92(2):305–312, 2018. Return to text.
- Martinez-Navarro, B., Pérez-Claros, J.A., Palombo, M.R., Rook, L., and Palmqvist, P., The Olduvai buffalo Pelorovis and the origin of Bos, Quaternary Research 68(2):220–226, 2007. Return to text.
- Oard, M., Australian marsupials: there and back again?, J. Creation 36(1):99–106, 2022. Return to text.
- Arment, C., To the Ark, and back again? Using the marsupial fossil record to investigate the Flood/post-Flood boundary, ARJ 13:1–20, 2020. Return to text.
- Anonymous, Small(er) is beautiful; museumvictoria.com.au, accessed 9 Oct 2023. Return to text.
- Buckley, M., Cosgrove, R., Garvey, J., and Prideaux, G.J., Identifying remains of extinct kangaroos in Late Pleistocene deposits using collagen fingerprinting, J. Quaternary Science 32(5):653–660, 2017. Return to text.
- Munro, N.D., Lebenzon, R., Sapir-Hen, L., Revisiting Late Pleistocene-Early Holocene mountain gazelle (Gazella gazella) body size change in the southern Levant: A case for anthropogenic impact, PLoS ONE 17(8), e0273024, 2022. Return to text.
- Pandolfi, L., Petronio, C., and Salari, L., Bos primigenius bojanus, 1827 from the early Late Pleistocene deposit of Avetrana (southern Italy) and the variations in size of the species in southern Europe: Preliminary Report, J. Geological Research, Article ID 245408:1–11, 2011. Return to text.
- Sen, S., Barrier, E., and Crété, X., Late Pleistocene dwarf elephants from the Aegean islands of Kassos and Dilos, Greece, Annales Zoologici Feenici 51(1–2):27–42, 2014. Return to text.
- Croitor, R., Bonifay, M.-F., and Bonifay, E., Origin and evolution of the Late Pleistocene island deer Praemegaceros (Nesoleipoceros) Cazioti (Depéret) from Corsicana and Sardinia, Bulletin of the Museum of Prehistoric Anthropology of Monaco 46:35–68, 2006. Return to text.
- van der Geer, A., Lyras, G.A., and Volmer, R., Insular dwarfism in canids on Java (Indonesia) and its implications for the environment of Homo erectus during the Early and earliest Middle Pleistocene, Palaeogeogrpahy, Palaeoclimatology, Palaeoecology 507(1552):168–179, 2018. Return to text.
- Bergmann, C., Ueber die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse (On the relationship between the heat economy of animals and their size), Göttinger Stud. 3:595–708, 1847. Return to text.
- Oard, M.J., An Ice Age Caused by the Genesis Flood, Institute for Creation Research, El Cajon, CA, 1990. Return to text.
- Bogin, B., Hermanussen, M., and Scheffler, C., Bergman’s rule is a ‘just-so’ story of human body size, J. Physiological Anthropology 41(1):1–13, 2022. Return to text.
- Oard, M.J., Frozen in Time, Master Books, Green Forest, AR, 2006 edn. Return to text.
- Or perhaps it was the other way around: perhaps greater longevity was the effect of larger body sizes. Return to text.
- Price, G.J., Louys, J., Cramb, J. et al., Temporal overlap of humans and giant lizards (Varanidae; Squamata) in Pleistocene Australia, Quaternary Science Reviews 125:98–105, 2015. Return to text.
- Wroe, S., A review of terrestrial mammalian and reptilian carnivore ecology in Australian fossil faunas, and factors influencing their diversity: the myth of reptilian domination and its broader ramifications, Australian J. Zoology 50:1–24, 2002. Return to text.
- Molnar, R.E., Dragons in the Dust: The paleobiology of the giant monitor lizard megalania, Indiana University Press, Bloomington, IN, 2004. Return to text.
- Klein, R.G., Why anatomically modern people did not disperse from Africa 100,000 years ago; in: Akazawa, T., Aoki, K., and Bar-Yosef, O. (Eds.), Neanderthals and Modern Humans in Western Asia, Plenum Press, New York, pp. 509–521, 1998. Return to text.
- Teske, P.R., Papadopoulos, I., McQuaid, C.D., Newman, B.K., and Barker, N.P., Climate change, genetics or human choice: why were the shells of mankind’s earliest ornament larger in the Pleistocene than in the Holocene?, PLoS ONE 7, e614, 2007. Return to text.
- Martini, P., Camel fossils from the El Kowm Basin, Syria. Diversity and evolution, Ph.D. dissertation, University of Basel, Switzerland, p. 162, 2019. Return to text.
- Gruber, K., Giant prehistoric kangaroos walked, not hopped; australiangeographic.com, 15 Oct 2014. Return to text.
- Carter, R., The extreme rarity of long-lived people in the post-Flood era, J. Creation 37(2):63–67, 2023. Return to text.
- Hebert, J., Rarity of long-lived post-Flood human fossils?, J. Creation 37(3):23–24, 2023. Return to text.





Readers’ comments
Comments are automatically closed 14 days after publication.